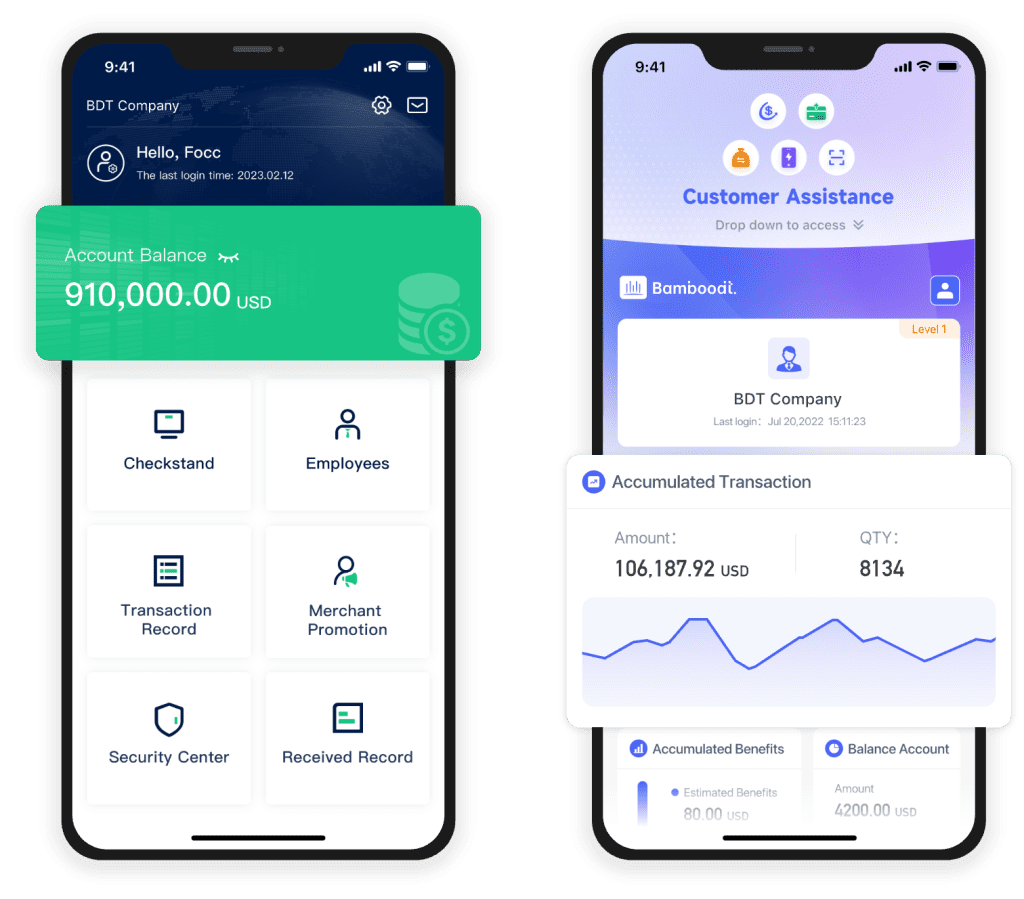
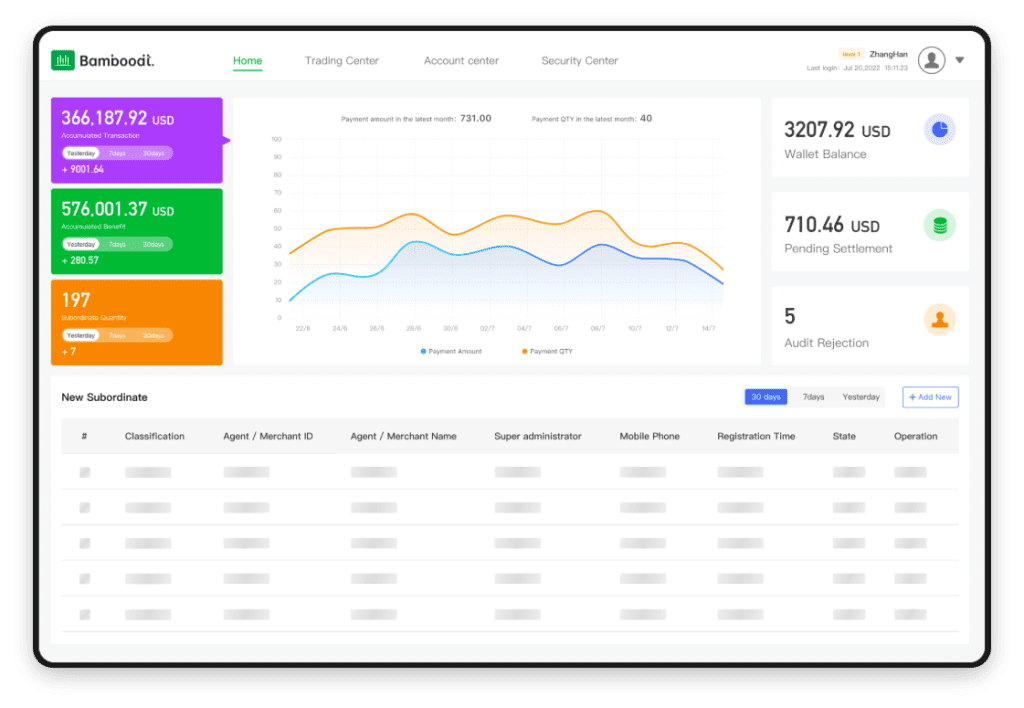
The pharmaceutical industry has witnessed dramatic transformations over the past few years, driven by the integration of advanced technology into every aspect of its operations. From drug discovery to clinical trials and even to patient engagement, custom software applications are playing a critical role. But what exactly makes software development for pharma unique, and how can companies harness this power to drive efficiency and compliance? In this article, we will explore the intricacies of advanced custom pharma software application development.
Understanding the Need for Custom Software in Pharma
The pharmaceutical landscape is deeply influenced by regulatory standards, research needs, and the demands of patients and healthcare providers. Off-the-shelf software solutions may not adequately address these unique requirements. Here’s why custom software development for pharma is essential:
- Compliance and Regulation: Pharma companies operate under stringent regulatory requirements from bodies such as the FDA and EMA. Custom software can be designed to include specific compliance features, ensuring that companies adhere to the law and avoid costly fines.
- Integration Capabilities: The ability to integrate with existing systems is vital. Custom software can ensure seamless data exchange between clinical trial management systems (CTMS), laboratory information management systems (LIMS), and electronic lab notebooks (ELN).
- Data Security: Protecting sensitive patient and research data is paramount. Custom applications can implement advanced security protocols tailored to the needs of each organization.
- Scalability: Pharma companies can face rapid changes in data volume during research phases. Custom solutions can be developed to easily scale as needed.
The Phases of Custom Pharma Software Development
Developing a custom software application requires a structured approach. Typically, it involves several key phases:
1. Requirements Gathering
This phase is about understanding the specific needs of users, stakeholders, and regulatory authorities. It involves interviews, surveys, and workshops.
2. System Design
In this phase, architects and developers outline the architecture of the system, including data flow, user interfaces, and external integrations.
3. Development
Here, developers create the application, focusing on quality code, usability, and compliance standards. Various programming languages and frameworks are utilized depending on the project.
4. Testing and Validation
Thorough testing is vital in ensuring that the software meets quality standards and compliance requirements. This includes unit testing, integration testing, and user acceptance testing (UAT).
5. Deployment
This phase involves releasing the software for use, including training users and providing documentation.
6. Maintenance and Support
Ongoing maintenance is crucial, especially in a highly regulated industry. Regular updates, security patches, and user feedback integration keep the software functioning optimally.
Key Technologies in Pharma Software Development
The landscape of technology is always evolving, and the pharma industry is no exception. Here are some of the technologies that are shaping advanced custom pharma software development:
- Artificial Intelligence (AI): AI can analyze vast datasets quickly, identifying potential drug candidates, predicting patient responses, and personalizing treatment plans.
- Blockchain: For ensuring data integrity and transparency in clinical trials, blockchain technology can help create immutable records of drug development stages.
- Cloud Computing: Cloud solutions facilitate collaboration among researchers and institutions involved in drug development, enabling real-time data access and sharing.
- Internet of Things (IoT): IoT devices can collect patient data remotely, which can be pivotal in clinical trials, providing real-time feedback on drug efficacy and patient adherence.
- Big Data Analytics: The pharmaceutical industry generates massive amounts of data. Advanced analytics tools help in interpreting this data for insights into drug development and market dynamics.
Challenges in Custom Pharma Software Development
Although there are significant benefits to custom development, challenges do exist. Below are some common hurdles faced by developers in the pharma sector:
- Regulatory Complexity: Navigating the complex regulatory landscape can be daunting, with ever-changing laws imposing restrictions on how software applications handle data.
- User Training: Employees must be trained to use new software effectively, which can involve both time and investment.
- Budget Constraints: Developing custom applications often requires significant upfront investment, which can be a barrier for smaller organizations.
- Data Migration: Transitioning from legacy systems to new applications can be fraught with challenges, including data loss or corruption if not handled carefully.
Future Trends in Pharma Software Development
The future of custom pharma software development is promising, with several trends shaping the industry:
- Personalized Medicine: Software solutions will support the growing trend towards personalized medicine, helping to tailor treatments to individual patient characteristics.
- Enhanced Security Protocols: With increasing data breaches, the focus on security in software development will continue to be paramount.
- Automation: Automating repetitive tasks such as data entry and report generation will free up resources for more critical work.
- Partnership and Collaboration: More partnerships will arise as companies collaborate to share data and technology, improving the research process.
The Importance of User-Centric Design
User-centric design is becoming increasingly important in software development for the pharmaceutical industry. Developers must focus on creating intuitive interfaces that allow for ease of use, especially when it comes to training staff and ensuring regulatory compliance. Engaging users during the development phase ensures that their feedback shapes the functionality of the software, leading to higher adoption rates and improved productivity.
Conclusion
As the pharmaceutical industry continues to evolve, so does the need for advanced custom software solutions. Meeting the unique requirements of this sector entails understanding regulatory pressures, technological advancements, and user needs. By investing in tailored software solutions, pharma companies can unlock efficiency, improve compliance, and ultimately enhance patient care.